Smoke toxicity is the greatest cause of death and of injury in unwanted fires, yet is completely unregulated outside of the mass transport industries. Simple assessment of smoke toxicity of commercial materials would provide the information designers and regulators need to avoid using the most dangerous products in places where fire deaths occur most often. This article describes
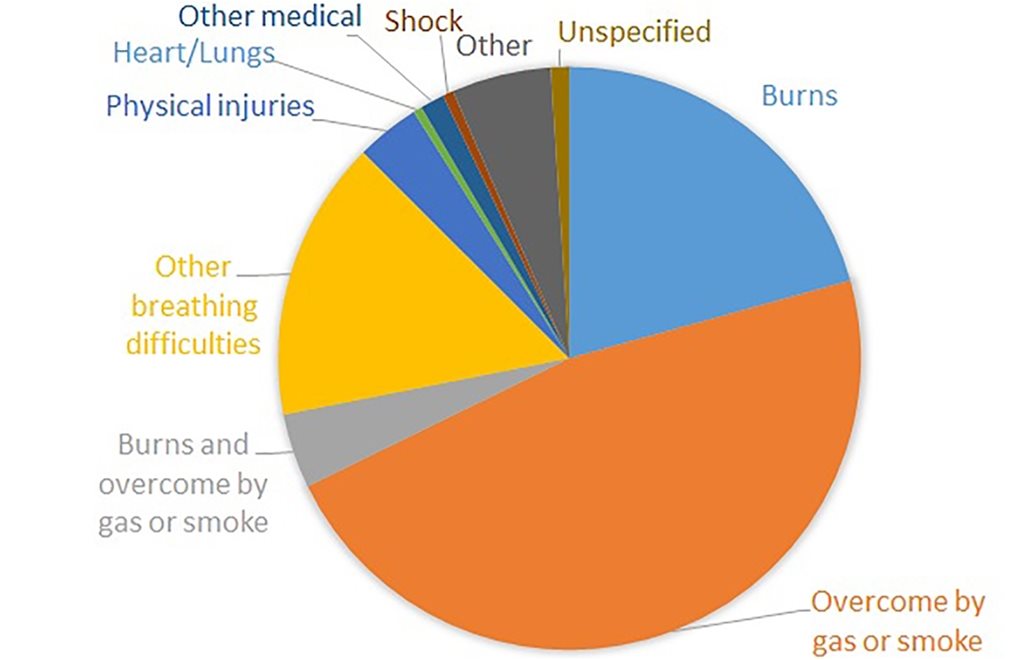
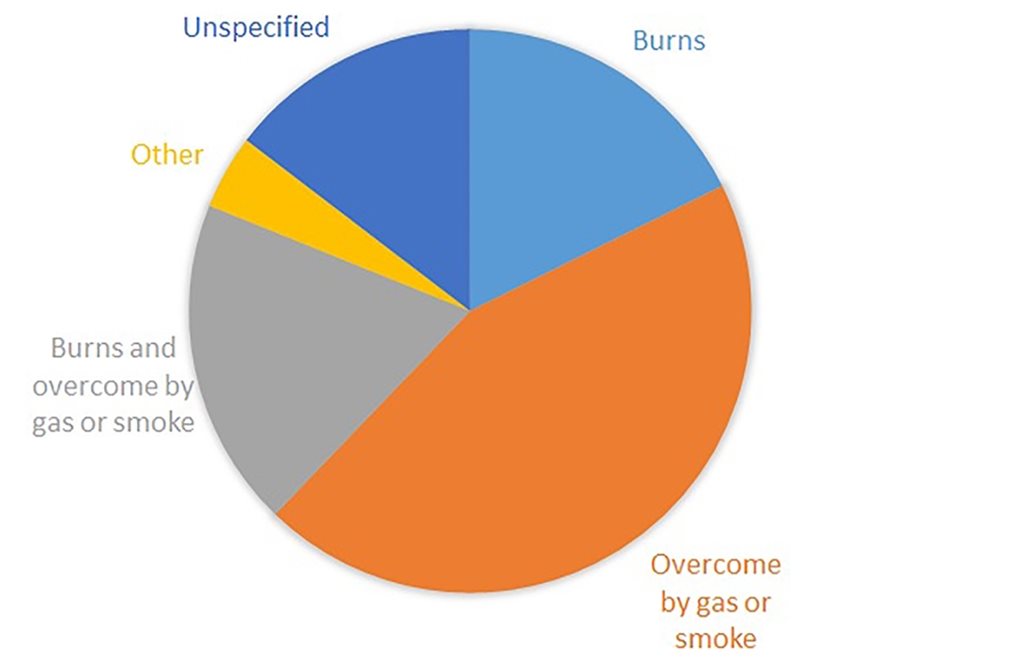
In the UK, as elsewhere, the majority of fire deaths and most fire injuries result from inhalation of toxic smoke. The cause of fatalities and injuries from UK dwelling fires 2018/19 is shown below.
The UK’s fire statistics show a progressive shift from burns to toxic gas inhalation as the leading causes of both death and injury, since they were first published in 1955. This can be attributed to the increasing proportion of synthetic plastics in the fuel of unwanted fires and to the increasing reliance on flame retardants to limit their flammability. Bulk synthetic polymers such as polyethylene, polypropylene, polystyrene and polyesters are more flammable than the wood, cotton and wool that they have replaced. It has been shown that gas phase flame retardants increase the toxicity of smoke.
It is this combination of greater flammability and increased smoke toxicity that has driven up the proportion of smoke toxicity deaths and injuries to burns. Flammability is fairly well understood and regulated in most high risk applications. In the UK this includes construction products, electrical and electronic goods, furniture and children's toys. Flammability covers a range of properties such as ignitability, flame spread and heat release. The usual worst case scenario for flammability is an atmospheric concentration of oxygen (O) of 21%.
Conversely, smoke toxicity tends to be lowest at an O concentration of 21%, when the main products are carbon dioxide (CO2) and water. In an enclosure fire, even with the windows and doors open, the ventilation rate is fixed and, provided there is sufficient fuel, the fire quickly becomes ventilation controlled. At this stage, the yields (masses of toxic products for a given mass of fuel) of carbon monoxide (CO) and hydrogen cyanide (HCN) can increase by factors between ten and 25, creating a highly toxic fire effluent. It has been established that this small, under ventilated stage of burning is responsible for the majority of fire deaths in the UK.
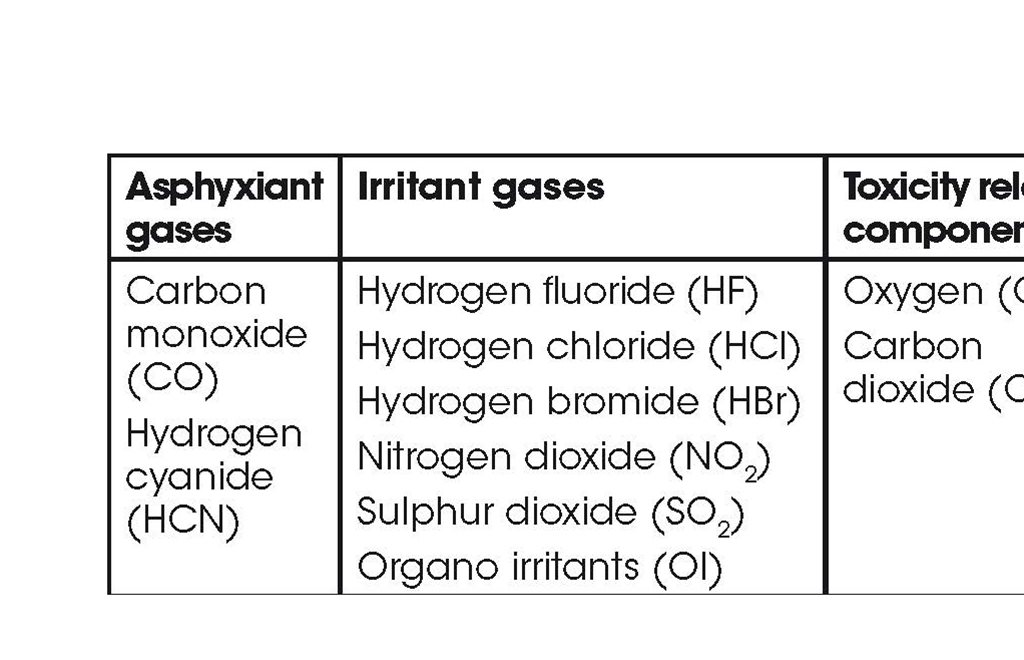
Asphyxiant and Irritant gases
Smoke contains asphyxiant gases (which prevent O uptake by the body, with loss of consciousness and ultimately death) and irritant gases (which cause immediate incapacitation, mainly by effects on the eyes and upper respiratory tract, and longer term damage deeper in the lung). The effect of asphyxiants and deep lung irritants depends on the accumulated doses – the sum of each of the instantaneous concentrations multiplied by the exposure time – for each product; upper respiratory tract irritation depends on the concentration alone.
The most common acutely toxic components in fire effluents are presented in the table below. Since every fire can be considered a unique chemical reactor, producing a mixture of known and unknown products, other components may make a greater contribution to the toxicity in a particular fire. However, the presence of other acute toxicants, which have yet to be characterised, is less likely than for longer term toxicants and carcinogens.
Oxygen and carbon dioxide
Fire effluents are O depleted, which causes impairment at around 15% O and lethality below 6%. However, in a fire effluent, the harm from other toxicants such as CO and HCN will be greater. CO2 is usually present at around 1 to 5% in smoke. Inhalation of CO2 increases breathing rate, resulting in increased uptake of O and of toxic gases.
Carbon monoxide
CO binds to the haemoglobin in red blood cells, impeding the transport of O within the body. It impairs an individual’s ability to escape causing at ten parts per million (ppm) impairment of judgement and visual perception; at 100ppm dizziness, headache and weariness; at 250ppm loss of consciousness; and at 1000ppm rapid death.
Hydrogen cyanide
HCN is approximately 25 times more toxic than CO. Inhaled cyanide is distributed throughout the extra cellular fluid of tissues and organs, where it inhibits the enzyme cytochrome oxidase, preventing the utilisation of O by the cells. The O deprivation caused by cyanide also increases the breathing rate in humans and primates, resulting in a factor of four increase in uptake of cyanide and other toxicants. Concentrations of 150ppm HCN cause collapse and loss of consciousness within a few minutes. In most cases, the unconscious victim will continue to breathe smoke until death occurs.
Irritant gases
Incapacitating irritants and smoke can prevent escape from fire, by causing severe pain to the eyes, nose, throat and upper respiratory tract. The effects range from tears and reflex blinking of the eyes; pain in the nose, throat and chest; breath holding; coughing; and excessive secretion of mucus, to bronchoconstriction and laryngeal spasms. At sufficiently high concentrations, or when attached to micrometre sized particles such as soot, most irritants can penetrate deeper into the lungs, causing pulmonary irritation which may result in post-exposure respiratory distress and death, generally occurring from a few hours to several days after exposure, due to pulmonary oedema (flooding of the lungs). This may trap the victim, allowing their uptake of other toxicants to continue. Victims trapped by irritant gases are likely to be found with elevated levels of CO in their blood, which will be reported as the cause of death.
Estimating acute smoke toxicity
In order to estimate the effects of the toxicant yields on an exposed human population, it is necessary to use measured data and established models of toxicity. The sum of the contribution of individual toxicants is usually expressed as a fractional effective dose (FED). This is the ratio of the amount of each toxicant present to the level causing a particular effect (incapacitation or death). A FED equal to one indicates that the effects of the individual components will result in incapacitation or death to 50% of the exposed population.
Preventing incapacitation, or ‘the inability to effect one's own escape’ is considered the critical point in fire safety in order to avoid fatalities. Incapacitation resulting from inhalation of the two asphyxiant gases found in fire effluents, CO and HCN may be predicted from an equation in ISO 135711. Over the timescales of fire exposure, CO’s effect is dose dependent (CO concentration multiplied by each exposure duration). The stimulation of breathing caused by cyanide is predicted by raising the concentration to the power 2.36.
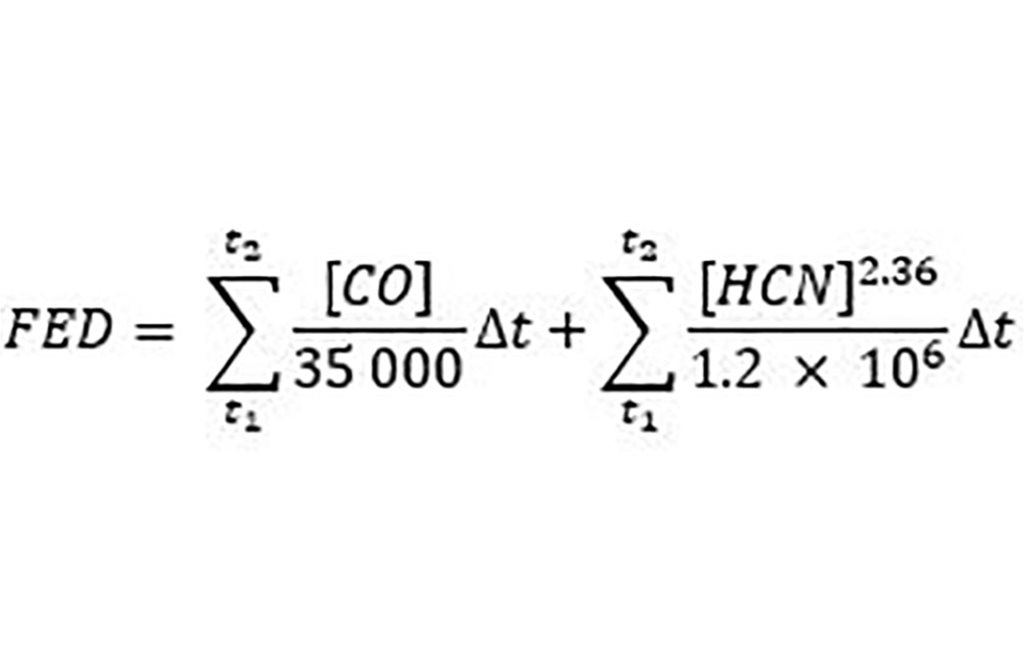
[CO] and [HCN] are the concentrations of CO and HCN in ppm, and ^t is the exposure time in min.
The combined effect of all irritant gases can be estimated from the fractional effective concentration (FEC) which is the sum of all irritant concentrations divided by the concentration of each irritant to incapacitate 50% of the exposed population (IC50, X).
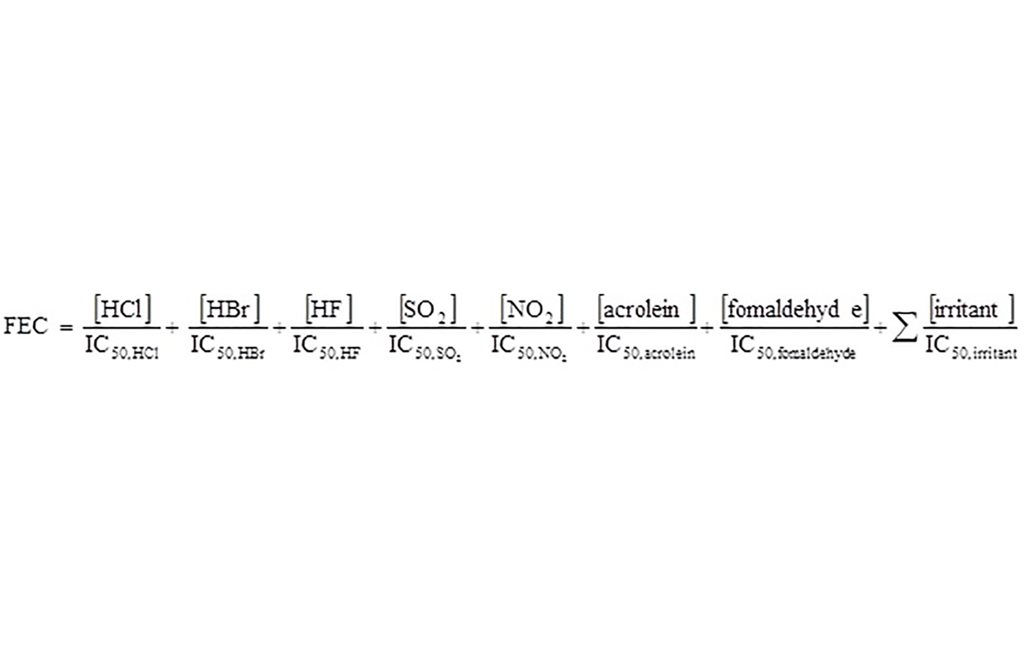
Alternatively, death can be predicted from rat lethality data using equations in ISO 133442. The predicted FED is the sum of the contributions from each toxic gas. The ratio of each toxicant concentration to its lethal concentration (LC50,X) is multiplied by a factor VCO2, to account for the increased breathing rate caused by CO2. The lethality model assumes exposure for 30 min to fixed concentrations of toxicants.
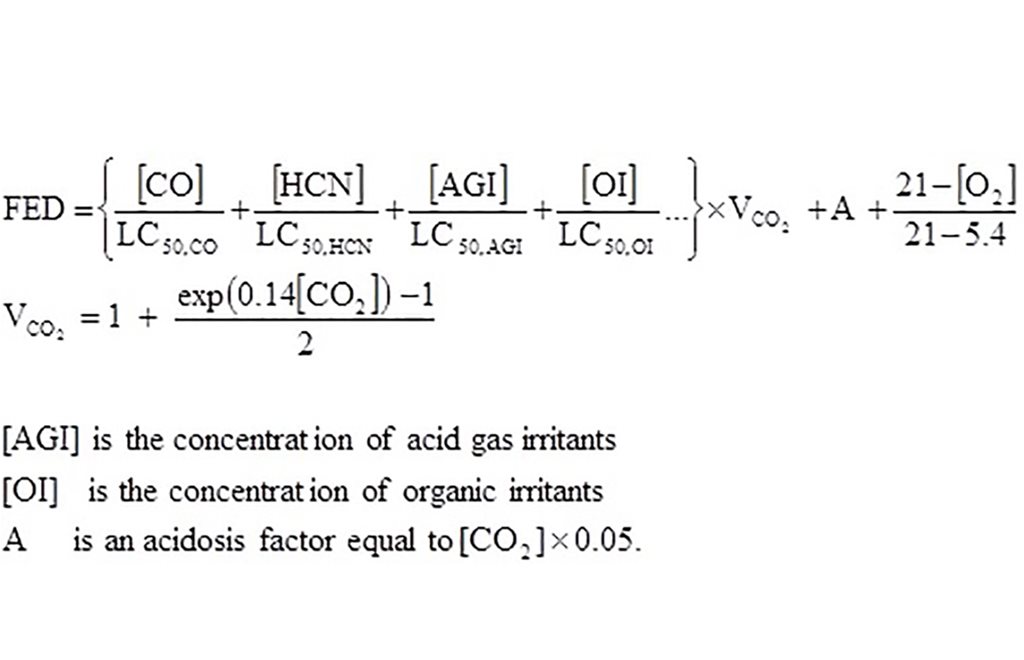
It is important to note that only n FED or FEC equal to one has a defined meaning. Smaller quantities of toxicants will have different effects. A FED equal to 2 would be sufficient to create a toxic atmosphere in twice the specified volume.
In order to have an intrinsic value for the fire effluent toxicity of a material, for example to determine the maximum permissible loading in a room or building, the FED can be related to the mass of material which would cause incapacitation or lethality for a given fire condition. The material-IC50, or material-LC50, is the specimen mass, M, of a burning material which would yield a FED of one in a volume, V, of 1 m3.
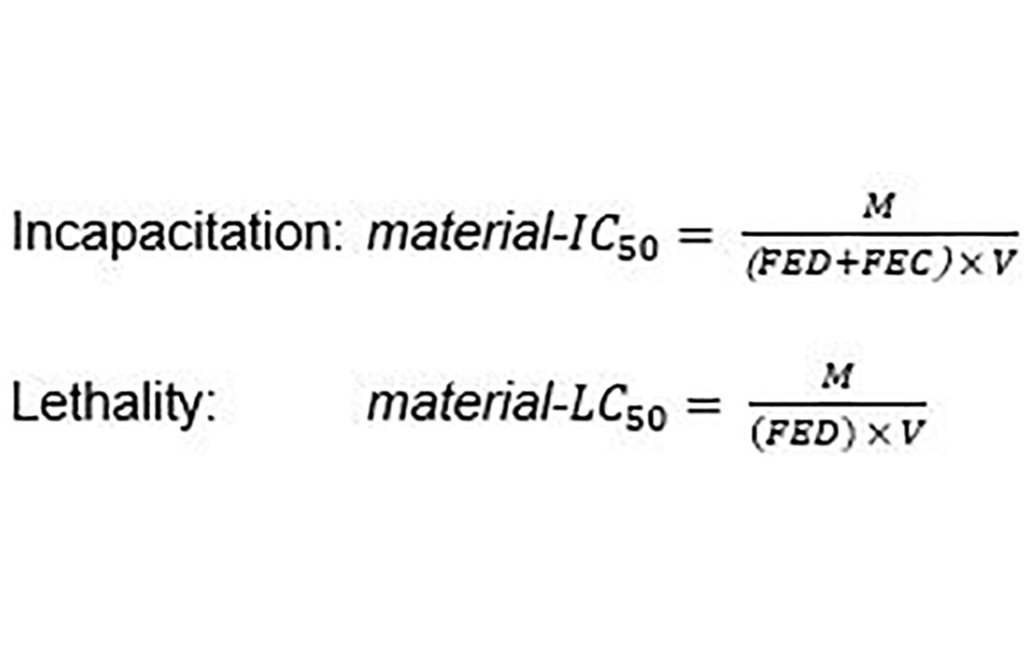
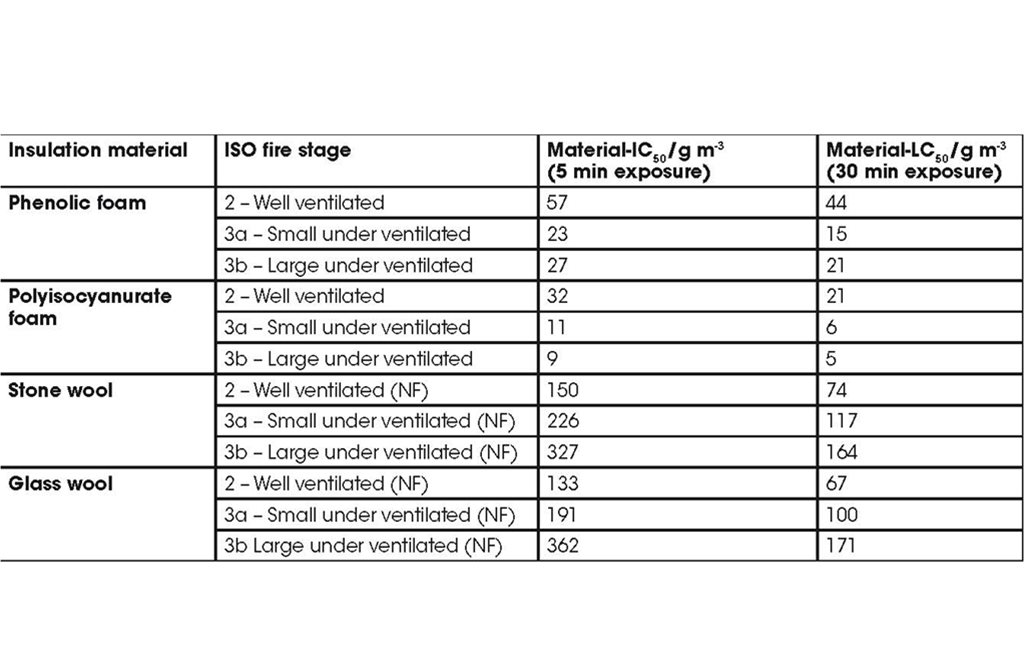
Comparing the smoke toxicities of different materials, the lower the material-IC50, or material-LC50, the smaller the amount of material necessary to cause incapacitation or lethality, the more toxic the material’s smoke will be. The values are referenced to the fire condition under which they were measured. As an example, the material-IC50, and material-LC50 were assessed for insulation products under three fire conditions. The values are provided below.
The table shows large differences between the smoke toxicity of different insulation materials whether they are assessed on their capacity to incapacitate or kill. It also shows a progressive increase in smoke toxicity (or decrease in material-IC50, and material-LC50) for the combustible foams with the transition from well ventilated to under ventilated flaming. As both the foams reported here contain gas phase flame retardants, their toxicity in well ventilated conditions is higher than may be expected.
Clearly neither incapacitation nor death are desirable outcomes. When assessing smoke toxicity, fire safety engineers need an additional factor to ensure that their building occupants will never be exposed to life-threatening fumes. In addition, allowance must be made for the parts of the population with greater susceptibility, for vulnerable occupants, the elderly, and those with underlying health problems, all of whom are likely to be present in a given occupancy or workforce.
Assessing toxic hazard from smoke
In the table below, the potential of a material to produce an incapacitating or lethal smoke is shown. It does not take into account its flammability. A flammable material will have a greater mass loss rate than a less combustible material. The toxic hazard depends on both the flammability of a material and the toxicity of its smoke. This could be expressed as:
toxic hazard = flammability x smoke toxicity
or, more precisely as: toxic hazard = mass loss rate x toxic product yield
Toxic product yield can best be quantified at fixed ventilation conditions. This allows the individual fire stages 2, 3a and 3b to be distinguished. The steady-state tube furnace (ISO 197003) generates toxic products under conditions of controlled ventilation, in effect at O concentrations from 5 to 21%. This forces combustion at any ventilation condition by allowing the heat flux on the sample to increase until steady burning is established. This provides a realistic assessment of toxic product yield over the full range of fire conditions. However, because the method is insensitive to the material’s flammability, to predict the toxic hazard, input of flammability data (usually measured at 21% O) is also required.
In Europe, flammability is measured using the single burning item test (SBI). A 30 kW propane burner is placed in an internal corner formed by the sheet material under test. The SBI combines surface spread of flame with penetrative burning into the bulk of the material, giving a fairly realistic indication of a material’s flammability. Penetrative burning can also be measured using a cone calorimeter, giving a direct measurement of mass loss rate. Both of these provide the required indication of the material’s flammability.
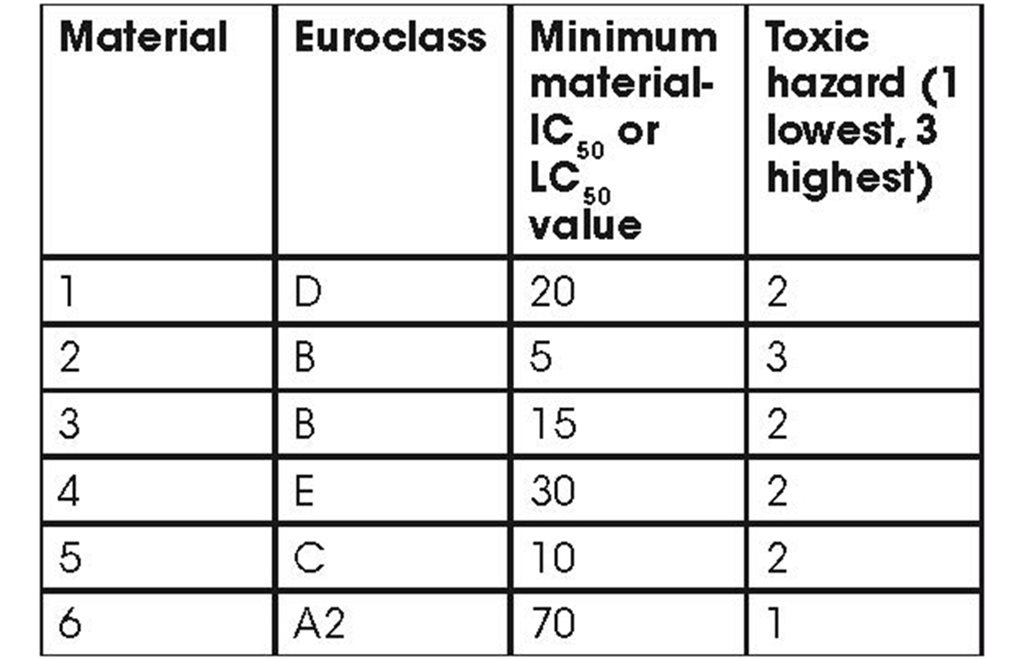
By combining the flammability and smoke toxicity, a simple hazard classification can be derived, for example, classifying materials as low, medium or high smoke toxicity. Materials posing the greatest hazard can then be identified and their use avoided in high risk or other sensitive applications. The Euroclass, measured using the SBI test gives a robust indication of flammability. In combination with the minimum value of either the five minute material-IC50, and or the 30 minute material-LC50, a simple toxic hazard classification can be derived. The table below illustrates this approach.
The following conclusions are drawn from the above:
- fire toxicity is the biggest cause of death and injury in fires, but is unregulated
- material composition has a big effect on fire toxicity
- combustible products drive fire growth and hence toxic gas production
- yields of CO and HCN increase dramatically as the fire grows
- assessing fire toxicity is easy and an essential component of fire hazard assessment
Professor T Richard Hull is professor of chemistry and fire science at the University of Central Lancashire
References
- ISO 13571: 2012: Life-threatening components of fire. Guidelines for the estimation of time to compromised tenability in fires, ISO, Geneva.
- ISO 13344: 2015: Estimation of the lethal toxic potency of fire effluents, ISO, Geneva.
- ISO/TS 19700: 2016: Controlled equivalence ratio method for the determination of hazardous components of fire effluents – Steady-state tube furnace, ISO, Geneva.